
Forest loss in Nigeria raises neonatal mortality and worsens birth outcomes by increasing malaria exposure among pregnant women.
Deforestation is an example of human activity, driven by the potential for economic benefit, that can lead to significant costs to human health. These health costs of environmental degradation are difficult to measure since they are often masked by increases in income that motivated cutting down trees in the first place. Mounting scientific evidence documents the negative ecological consequences of forest loss – increased greenhouse gas emissions, biodiversity loss, soil erosion, disruption of water cycles, etc. There is a more recent, but growing, body of work examining the impacts of forest loss and forest degradation on human health (Garg 2019, Carrillo et al. 2019).
The ecological and human behavioural changes triggered by forest loss drive increased disease incidence
One major way forest loss impacts human health is by increasing the risk of vector-borne infectious diseases, such as malaria, caused by Plasmodium parasite species and transmitted to humans through the bites of infected female Anopheles mosquitoes. Forest loss leads to ecological changes, including elevated ground temperatures, increased puddle formation, and the disappearance of species that prey on mosquitoes. It also triggers human behavioural changes, like increased contact between people and mosquitoes due to expanding agricultural activities and settlement locations. Together, these factors can contribute to a rise in malaria incidence (Pattanayak et al. 2006; Yasuoka and Levins 2007). A survey of published field studies on 87 mosquito species across 12 countries reveals that deforestation is a significant human-driven factor affecting malaria prevalence. In deforested habitats, 53% of mosquito species are more prevalent, of those 57% are carriers of human pathogens (Burkett-Cadena and Vittor 2018).
This increased malaria incidence has devastating consequences – in high-transmission areas in the tropics, malaria is of perpetual concern and, if left untreated within 24 hours, can develop into severe illness and even death. Partial immunity to malaria is acquired during childhood; yet very young children who are yet to acquire immunity and pregnant women who are exposed to both maternal and placental malaria are at considerable risk. While pregnant women with malaria are often asymptomatic, malaria exposure during pregnancy often results in prematurity and low birthweight, and also increases the risk of neonatal mortality (Brabin et al. 2004; Desai et al. 2007). Longer-term consequences of childhood malaria exposure includes negative impacts on education, fertility, and productivity (Cutler et al. 2010; Barreca 2010; Lucas 2013).
Nigeria carries the world’s largest malaria burden and has experienced extensive forest loss
We study the impacts of deforestation-induced malaria incidence in the 21st century Nigeria (Berazneva and Byker 2017; 2024). Nigeria is home to one-fifth of Africa’s population and carries a disproportionate malaria burden: it accounts for 19% of global malaria deaths (WHO 2018). The country has also lost over 17 million hectares of forest and other wooded land area since 1990, with an average annual forest loss of 3.5% (FAO 2015). This loss and forest degradation more generally are attributed to conversion of forest areas to agriculture, urbanisation, official and unofficial logging and fuelwood collection, but also uncoordinated land use policy and weak enforcement (Usman and Adefalu 2010).
Establishing the link between forest loss and malaria
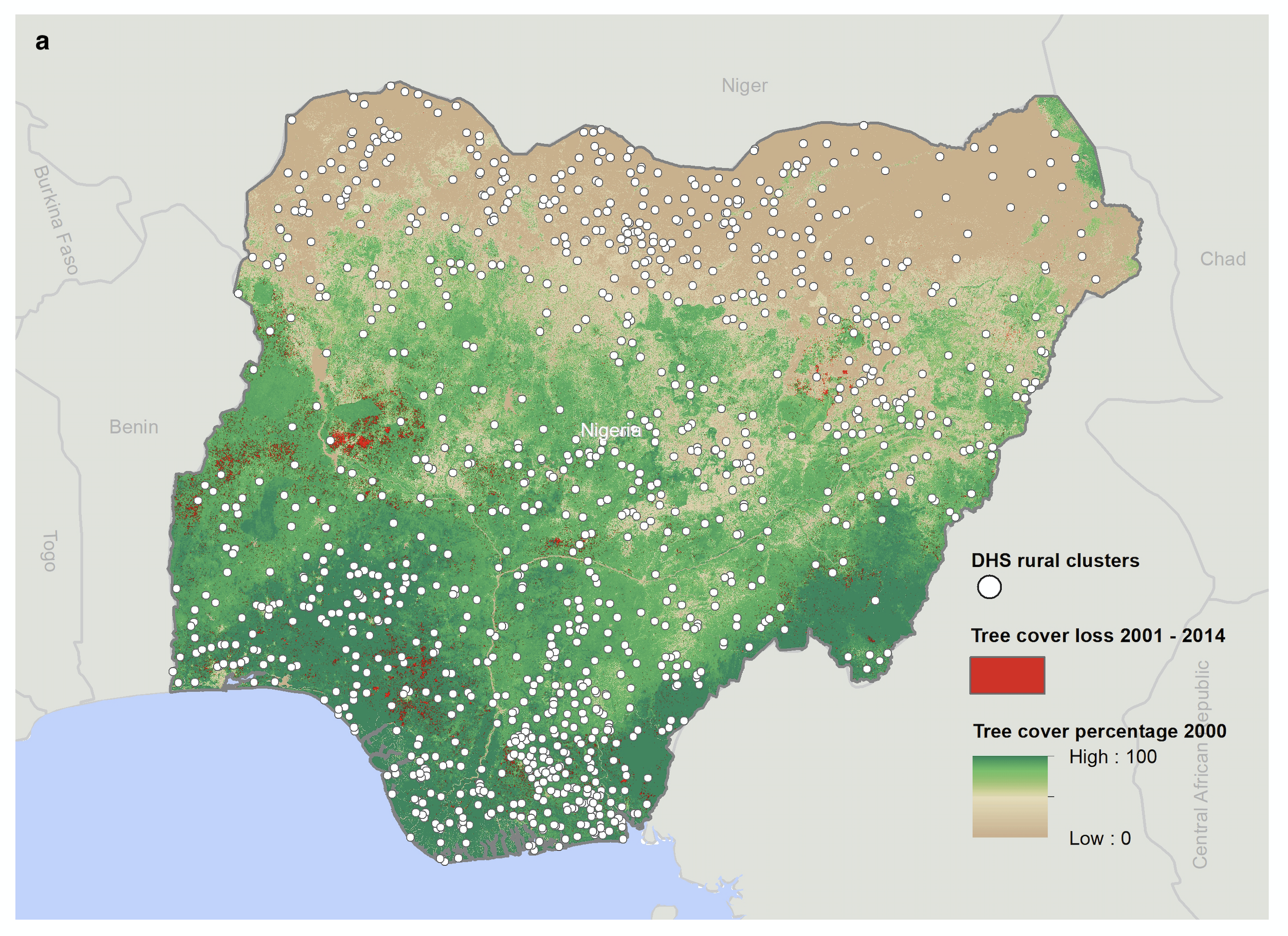
As Figure 1 illustrates, we geolink a global high-resolution panel of forest loss data (Hansen et al. 2013) with child- and mother-level information from several waves of the nationally representative Nigeria Demographic and Health Surveys (DHS) and Malaria Indicator Surveys (MIS). Our data encompass child health outcomes and complete birth and mortality histories for all live births occurring in the five years prior to the DHS surveys, enabling us to link both leads and lags of forest loss to the year of birth and the year of interviews. This allows us to identify “critical periods” of exposure to forest loss-induced malaria. Since forest loss is potentially nonrandom and there are other economic trends that may be associated with health outcomes, in order to provide causal estimates, we focus on variation in forest loss within small geographic areas. We also examine outcomes for children born to the same mother in different years exposed to different levels of tree loss using a mother-fixed effects specification.
Figure 1: Forest loss and DHS data.
Forest loss increases malaria incidence in children in rural Nigeria
We find the strongest link between forest loss one year ago and malaria incidence in the year of the interview – one standard deviation (about 0.2 square km) of forest loss leads to over 5% increase in incidence of malaria – and this result holds both when we measure malaria incidence by mother’s report of fever (as in DHS) and direct tests for malaria infection using rapid diagnostic tests and microscopy (as in MIS). This one-year delay in impact is consistent with public health literature (Pattanayak et al. 2006) that expects ecological and human behaviour changes after forest loss take time to develop. To provide further evidence that forest loss is specifically causing malaria, rather than a general impact on health, we test for and do not find any impact of forest loss on two other infectious diseases in young children (diarrhea and acute respiratory diseases) which are not associated with forest loss.
Forest loss increases neonatal mortality and worsens birth outcomes for surviving children
We find that in utero exposure to malaria results in increased neonatal mortality: one standard deviation of forest loss (about 0.2 square km) of forest loss leads to a 9-15% increases in the likelihood of death within the first month of life. The impact of malaria exposure in utero on post-neonatal mortality (during the second through 12th month after birth) is less pronounced. This result is consistent with the evidence that negative prenatal conditions manifest soon after birth. Malaria-related low birthweight is estimated to account for more than 11% of neonatal deaths and 6% of all infant deaths in malaria-endemic regions of Sub-Saharan Africa (Guyatt and Snow 2001). We also find lower weight-for-age, lower height-for-age, and increased likelihood of being stunted due to in utero malaria exposure amongst surviving children in our sample. This is consistent with evidence of reduce birth weight of children in Brazil whose mothers were exposed while the child is in utero to dengue fever, another prevalent mosquito-born disease.
To put these health impacts in context, with some additional assumptions, we calculate that over 14,000 neonatal deaths per year can be attributed to forest loss and the consequent increased malaria incidence in Nigeria. The yearly neonatal mortality costs amount to over $14 billion (if we assume $1 million as a value of a statistical life, as in Jayachandran (2009)). These estimates are lower bound of the health costs of forest loss in Nigeria, since they do not include impacts on surviving children.
Policy makers need to consider these health costs of forest loss
The substantial health costs we identify must be included in any policy or programme aimed at forest conservation and reducing forest loss to get us closer to true social costs of deforestation. Compared to existing research that examines outcomes later in life, our focus on in utero exposure (when even pregnant mothers may not be aware of the infection) and infants identifies a cost of deforestation net of potential compensating behaviours or economic benefits of forest loss that may accrue later in life. In addition, our results offer evidence for health interventions targeted to often most vulnerable populations – infants and pregnant women – which could include distribution of insecticide-treated nets and anti-malaria drugs, malaria vaccine, use of intermittent preventive treatment of malaria during pregnancy, and indoor residual spraying to prevent malaria.
References
Barreca, A I (2010), “The long-term economic impact of in utero and postnatal exposure to malaria,” The Journal of Human Resources, 45(4): 865–892.
Berazneva, J, and T S Byker (2017), “Does forest loss increase human disease? Evidence from Nigeria,” American Economic Review, 107(5): 516–521. https://doi.org/10.1257/aer.p20171132.
——— (2024), “Impacts of environmental degradation: Forest loss, malaria, and child outcomes in Nigeria,” The Review of Economics and Statistics, 106(5): 1254–1267. https://doi.org/doi.org/10.1162/rest_a_01238.
Brabin, B J, C Romagosa, S Abdelgalil, C Menéndez, F H Verhoeff, R McGready, K A Fletcher, et al. (2004), “The sick placenta—the role of malaria,” Placenta, 25(5): 359–378. https://doi.org/10.1016/j.placenta.2003.10.019.
Burkett-Cadena, N D, and A Y Vittor (2018), “Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens,” Basic and Applied Ecology, Insect Effects on Ecosystem Services, 26 (February): 101–110. https://doi.org/10.1016/j.baae.2017.09.012.
Carrillo, B, D K Branco, J C Trujillo, and J E Lima (2019), “The externalities of a deforestation control policy in infant health: Evidence from Brazil,” Economic Development and Cultural Change, 67(2): 369–400. https://doi.org/10.1086/698164.
Cutler, D, W Fung, M Kremer, M Singhal, and T Vogl (2010), “Early-life malaria exposure and adult outcomes: Evidence from malaria eradication in India,” American Economic Journal: Applied Economics, 2(2): 72–94.
Desai, M, F O ter Kuile, F Nosten, R McGready, K Asamoa, B Brabin, and R D Newman (2007), “Epidemiology and burden of malaria in pregnancy,” The Lancet Infectious Diseases, 7(2): 93–104.
FAO (2015), Global Forest Resources Assessment 2015, Food and Agriculture Organization of the United Nations (FAO), Rome, Italy. Available at: http://www.fao.org/forest-resources-assessment/en/.
Garg, T (2019), “Ecosystems and human health: The local benefits of forest cover in Indonesia,” Journal of Environmental Economics and Management, 98 (November): 102271. https://doi.org/10.1016/j.jeem.2019.102271.
Guyatt, H L, and R W Snow (2001), “Malaria in pregnancy as an indirect cause of infant mortality in Sub-Saharan Africa,” Transactions of The Royal Society of Tropical Medicine and Hygiene, 95(6): 569–576. https://doi.org/10.1016/S0035-9203(01)90082-3.
Hansen, M C, P V Potapov, R Moore, M Hancher, S A Turubanova, A Tyukavina, D Thau, et al. (2013), “High-resolution global maps of 21st-century forest cover change,” Science, 342(6160): 850–853.
Jayachandran, S (2009), “Air quality and early-life mortality: Evidence from Indonesia’s wildfires,” Journal of Human Resources, 44(4): 916–954. https://doi.org/10.3368/jhr.44.4.916.
Lucas, A M (2013), “The impact of malaria eradication on fertility,” Economic Development and Cultural Change, 61(3): 607–631. https://doi.org/10.1086/669261.
Pattanayak, S K, K Dickinson, C Corey, B Murray, E Sills, and R Kramer (2006), “Deforestation, malaria, and poverty: A call for transdisciplinary research to support the design of cross-sectoral policies,” Sustainability: Science, Practice, & Policy, 2(2). Available at: http://search.proquest.com/docview/1428624299/abstract/4A65B427C2334A33PQ/1.
Usman, B A, and L L Adefalu (2010), “Nigerian forestry, wildlife and protected areas: Status report,” Biodiversity, 11(3–4): 54–62.
WHO (2018), World Malaria Report 2018, World Health Organization (WHO), Geneva. Available at: http://www.who.int/malaria/publications/world-malaria-report-2018/report/en/.
Yasuoka, J, and R Levins (2007), “Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology,” The American Journal of Tropical Medicine and Hygiene, 76(3): 450–460.


